TEACHING MATERIALS for Students
2. New technologies for textile functionalization
2.3. Sol-gel process
The sol-gel process is a chemical synthesis method where an alkoxide network is created by progressive condensation reactions of molecular precursors from a liquid medium.
The sol-gel process to produce hybrid coatings, compared to other methods, has several advantages. This process is often classified as a “green” technology since it uses compounds that do not introduce impurities into the end product (e.g., ceramic processing), is waste-free, excludes the stage of washing, and the processing temperature is generally low, frequently close to room temperature.
The first step to implement a synthesis through the sol-gel method is to select the chemical compounds, known as the precursors (see Figures below) that are combined forming a sol. This term designates a stable suspension of colloidal particles within a liquid. This first step is a typical chemical transformation aiming the formation of a sol of colloidal particles or a solution of oligomers (small polymers). As sol is a fluid, it can be cast in a mold, or be applied on a surface using various shaping techniques, e.g., spraying on a surface, dipping, or spinning through a set of rotating nozzles. As the sol is a relatively stable solution it can be stored for a certain time before further casting, or applied by any of the above referred methods. Spraying and electrodeposition processes emerged recently and could be the major sol-gel coating application methods in the near future.
Fig. 2.3.1 Sol-gel coatings Fig. 2.3.2 Precursors used in the synthesis of hybrid materials
To achieve gelation, the chemical transformations of the sol in gel must be allowed to proceed
until a single and interconnected network forms, only limited by the walls of the container and the
mass of the reactive mixture. Another step in sol-gel art is drying, which is a very critical step. In many instances, a dry gel needs further thermal treatment to be densified.
There are two main methods to prepare sol-gel coatings: the inorganic method and the organic one. The first method involves the evolution of networks through the formation of a colloidal suspension (habitually, oxides) and gelation of the sol (colloidal suspension of very small particles that may vary from 1 to 100 nm) to form a network in a continuous liquid phase.
The most commonly used method to prepare sol-gel coatings is the organic one, which normally starts with a solution of monomeric metal or metalloid alkoxide precursors M(OR)n in an alcohol or other low-molecular weight organic solvent. M represents a network-forming element, such as Si, Ti, Zr, Al, Fe, B, etc., and R is typically an alkyl group (CxH2x+1). This later approach has the advantage of obtaining organic-inorganic hybrid materials combining the behavior of the organic component (polymeric) with the stiffness of the inorganic (alkoxide) backbone.
Generally, the sol-gel method used to obtain hybrid materials (organic method) occurs in four stages:
(i) a hydrolysis reaction of the metal alkoxide groups (–OR) are replaced by the hydroxyl groups (–OH);
(ii) condensation and polymerization of monomers to form chains and particles;
(iii) growth of the particles; and
(iv) agglomeration of the polymer structures followed by the formation of networks that extend throughout the liquid medium resulting in a gel.
Indeed, both the hydrolysis and condensation reactions occur at the same time once the hydrolysis reaction has been initiated. Both the hydrolysis and condensation steps produce low molecular weight by-products, such as alcohol and water.
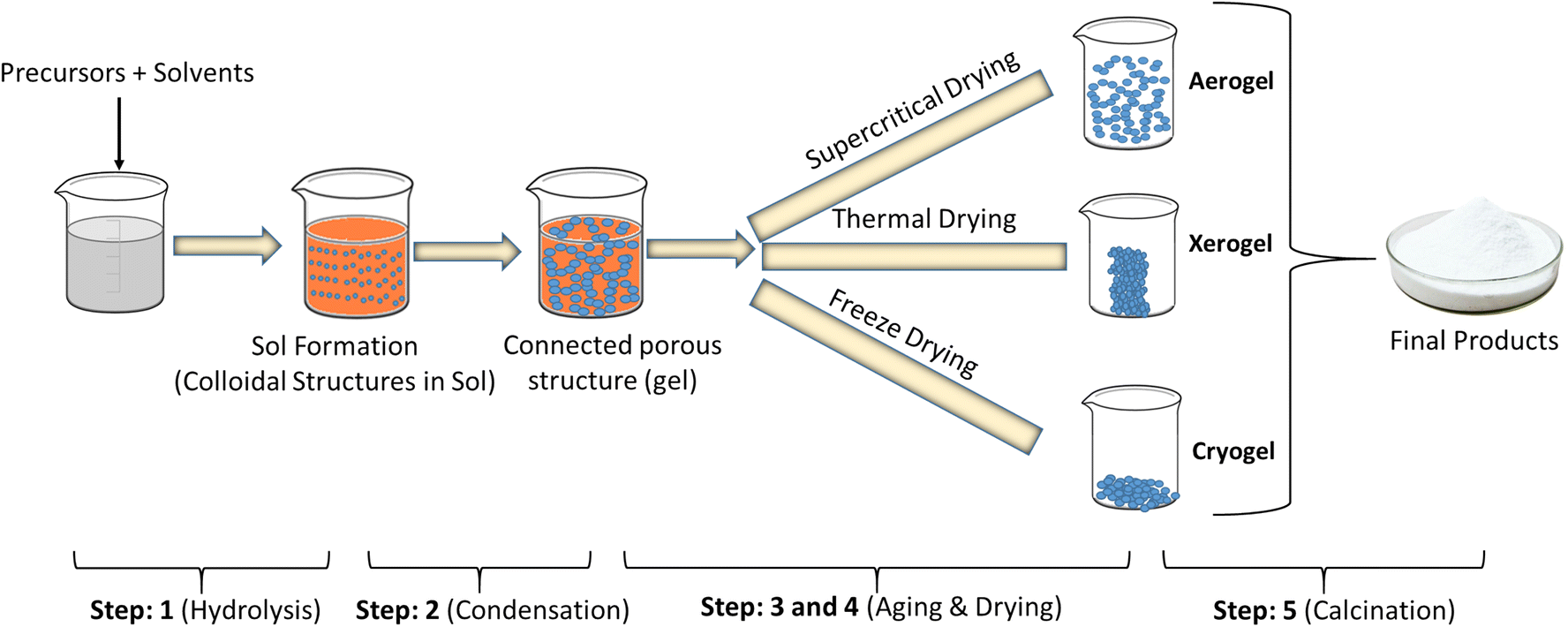
Fig. 2.3.3 Steps to obtain hybrid materials (organic method) occurs in sol-gel method
Field of applications of sol-gel coatings, examples of some will be persented in chapter 3.
Source:
Rita B. Figueira et al.: Coatings 2016, 6, 12; doi:10.3390/coatings6010012
Parashar, M. et al.:: J Mater Sci: Mater Electron 31, 3729–3749 (2020)